A sustainable energy system can only be based on renewable energy. There is no choice in the matter. It is not a question whether we like it or not or whether we feel that our energy needs can be adequately met by renewables. To be sustainable, we have no option but to develop systems that enable us to keep our energy needs low enough so that they can be met by renewable supplies.
This is the context within which this paper discusses building a hydrogen economy. Hydrogen produced from finite energy resources would not solve anything satisfactorily. It has to be part of an integrated sustainable energy strategy.
1. WHY HYDROGEN?
Here are some of the reasons for using hydrogen as part of an integrated energy strategy:
- We will need a carbon-free fuel for mobile applications in the mid-term future. It should have started becoming available before 2010.
- The European automotive industry has committed itself to reducing the average CO2 emission of the entire European car range on sale in 2008 to 140 g/ km and to 120 g/km in 2012. If this target is not met, the European Commission will enforce it legally. At present, car manufacturers do not know how they can achieve this goal using existing propulsion technologies and conventional fuels while maintaining the present mix of vehicle classes.
- Hydrogen offers the most promising perspective as a fuel for mobile applications, since it can be produced from various sources such as conventional electricity, renewable electricity, conventional steam reforming and the regenerative steam reforming of the products of a biomass gasification process. Hydrogen has the highest energy source flexibility of any alternative fuel and therefore offers the most robust strategy for supplying a clean transportation fuel
- Hydrogen can be used for many purposes ranging from burning for heat as gas, through chemical processes to its conversion to electricity in fuel cells
- Hydrogen, via electrolysis and fuel cells, provides a way of storing large quantities of electricity without running into the raw materials limitations that batteries would present. It can therefore be used as buffer storage to meet peak power demand. Within the next five years it is likely to be used for storing off-shore wind energy and in smaller, stand-alone power systems based on renewable energies. In the longer term - after 2020 - I expect it to be used for large scale applications.
Hydrogen cannot solve the energy problem, but it can help to switch the transport sector smoothly from fossil fuels to renewable ones. In addition, hydrogen can create links between different energy carriers, e.g. between electricity, transport fuel and gas, thus linking heat energy supply with fuel supply and with electricity production.
2 COMPONENTS OF A HYDROGEN ECONOMY
2.1 METHODS OF PRODUCTION
Hydrogen emits almost no pollution when it burns so its environmental benefits are determined by the way it is produced. World production is already equivalent to 20-25% of annual natural gas production The following methods are generally used:
1. As a chemical by product - Hydrogen is an unavoidable by-product during the production of chlorine, acetylene, styrene or cyanide. It is also produced in petrochemical cracking processes such as the catalytic reforming and cracking of crude oil during upgrading or ethylene production. However, the purity of the hydrogen produced in these ways varies from more than 99.5 percent (chlorine synthesis) to below 60 percent (ethylene production). Even the city gas produced in the last century from coal contained about 50 percent hydrogen.
Most of this hydrogen is mixed with natural gas and burned close to where it was made to produce process heat. However, during the introduction of hydrogen-fuelled transport, it could become available at reasonable cost to supply the first fuelling stations.
2. From fossil fuels - Almost half of today's hydrogen demand is supplied by steam reforming natural gas or, in minor quantities, by the partial oxidation of oil products. Most of this production takes place close to the site on which the gas will be used in order to avoid having to transport it. Steam reforming is usually the cheapest way to produce hydrogen and nearly all the gas used for desulphurisation at refineries, for ammonia production for fertilisers, or for methanol synthesis is produced this way. About 30 percent of the energy content of the natural gas input is lost in the conversion.
3. With electricity - Another well-established method of hydrogen production is electrolysis - splitting water into oxygen and hydrogen gas. This is the method of choice where cheap electricity is available. Norway has used this method in its fertiliser plants for almost 80 years. Electrolysers are highly modular and can range from very small units producing a few cubic metres of hydrogen per hour - or several kW electric power demand - up to the multi megawatt level. Today's modern electrolysers are optimised to produce hydrogen at a pressure of 30 bar and are designed to cope with a fluctuating electrical power input.
The attraction of electrolytic hydrogen is that its production can be on a small scale, is simple technically, quiet and pollution-free, and gives wide freedom of choice for the primary energy input. It enables hydrogen to be produced close to or apart from the site of the electricity production, from fossil fuels as well as from renewable sources. This feature is ideal for a smooth and steady transition from fossil- to renewables-generated hydrogen.
The various methods of producing hydrogen have very different economic and ecological characteristics. For example, electrolytically produced hydrogen from coal-fired power plants would result in CO2 emissions of about 2 kg/kWh or 6 kg/m3. For comparison, gasoline emits about 0.27 kg/kWh during combustion. On the other hand, hydrogen from wind or solar electricity would be almost free of polluting emissions.
Although electrolysis using electricity from intermittent renewable sources involves higher amortisation costs because the plant involved is not able to produce constantly throughout the year, it can still provide cheap hydrogen. For instance hydrogen from large scale hydropower can cost between 2.5 - 5 cents/kWh or 7.5-15 cents/cubic metre. For comparison, consumers in Europe pay about 10 cents per kWh of gasoline, or 1 Euro per litre, including tax.
Production using power from large offshore wind farms would cost about 10-15 cents/kWh or 30 - 45 cts/m3. This is roughly in the same range as hydrogen produced from geothermal electricity, assuming that modern low temperature geothermal power generation (hot dry rock, organic rankine cycle) results in electricity cost of below 10 cents/kWhel. The higher electricity production costs are outweighed by the advantage of 8,000 operating hours per year for the electrolyser, instead of about 2,000-3,000 hours per year for wind energy converters.
It takes, on average, about 4 kWh of electricity to produce 1 cublic metre or 3 kWh hydrogen as 20 -25 percent of the energy is lost in the conversion process. Accordingly, the first use of renewable electricity should not be for electrolysis but to replace fossil fuels (predominantly coal) in stationary applications. The electrolytic production of hydrogen could, however, be considered in circumstances like these:
- If electricity is best produced without reference to immediate consumer demand. The surplus electricity can easily be used for hydrogen production.
- If offshore wind energy parks are far from land. Connecting their fluctuating electricity production to the grid directly might be more costly than producing hydrogen at the wind park and piping it to the final destination to satisfy electrical and transport fuel demand.
- If an island is not coupled to the national grid and needs an electricity storage system to become completely independent of outside energy sources. Above a certain size, hydrogen storage is more economic and more ecological than using batteries. Of course, fossil fuel storage would be the cheapest option if one ignored possible supply restrictions CO2 emissions and external costs.
- Logically it might be best use renewable electricity first to replace fossil electricity completely and only when this is done and huge storage problems arise, to convert the surplus into hydrogen. But realistically there are different players with different interests within different demand sectors and the transport sector urgently needs to escape from its oil dependence and the environmental damage that that does. In the early phases of building a hydrogen economy there will therefore inevitably be some straying from the optimum pathway. However, as long as each individual step fits into the overall scheme, it is worth taking it even under sub-optimal conditions.
4. From biomass - Using hydrogen from biomass gasification in a fuel cell is a more efficient way of producing electricity than burning the biomass in a thermal generator, even on a small scale. Several options are open. If ten percent of the land in a district is converted to fuel production, biomass harvesting within a radius of 25 km would feed a 5 MW gasification plant. The crude biogas (CO, H2) could then be fed into a high temperature molten carbonate fuel cell which produces both electricity and heat at about 4500C in sufficient quantities to supply about 3000 houses with heat and electricity. There would also be enough hydrogen to fuel 17 buses with a daily range of about 300 km.
This system has yet to prove itself in practice
but all its components are known and technically
feasible, though not yet optimised for this
use. A prototype would cost close to €10 million
to build and then €2 million per year to run.
This would cover everything from planting the
biomass, the gasification and purification plant,
the fuel cell, hydrogen compression and building
a transport fuelling station. After a few
years' experience, it ought to be possible to
reduce the construction and operating costs by
about 30 percent. The plant would deliver about
4 million kWh of electricity per year and 16
million cubic metres of hydrogen plus 4 million
kWh of heat. If the electricity was sold at 10
cts/kWhel and the hydrogen fuel at &8264;1 per litre
gasoline equivalent, the annual return would
total about €5 million. Biomass gasification
offers hydrogen conversion efficiencies somewhat
lower than from natural gas reforming, of
the order of 60 - 65 percent.
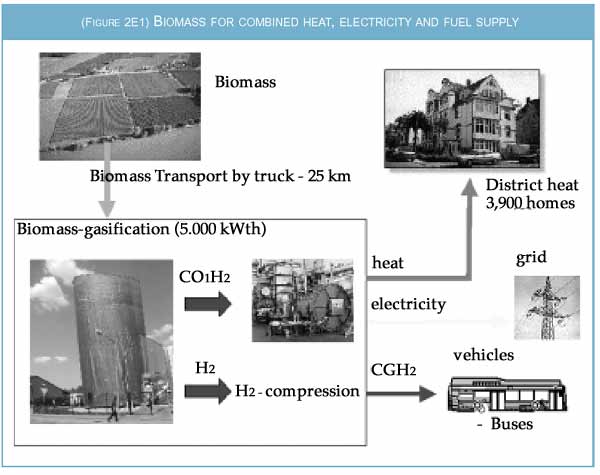 |
| Figure 2E1: Sketch of a biomass gasification plant supplying heat, electricity and transport fuel from agricultural sources such as wood or dedicated crops. |
5.By other methods - Other production methods like the direct generation of hydrogen by algae or bacteria are already carried out on laboratory scale. We can therefore move towards a hydrogen economy immediately, confident that the future will offer a broader range of hydrogen sources than we know at present.
2.2 STORAGE AND TRANSPORTATION
In principle, hydrogen could be used in mobile applications today but storage improvements are desirable to increase its acceptability for powering vehicles. Two storage methods, liquefaction and compression, have been used in industry for many years. Both have advantages and drawbacks. Liquid hydrogen offers higher energy densities - 2.36 kWh per litre - for long distance transport and for on board storage in automobiles. This is approximately one quarter of the energy density of gasoline fuel. One litre of hydrogen weighs about 70g whereas its energy equivalent, a quarter litre of gasoline, weighs about 200g. However, in order to liquefy hydrogen, the gas must be cooled to - 250 0C. This process consumes about 14 kWh primary energy per kg of hydrogen in today's liquefaction plants with throughput of 4.4 tons per day. This is about 40 percent of the hydrogen's energy content. Theoretically this figure could be improved by a factor of three in energetically optimised liquefaction plants.
Another serious drawback is that liquid hydrogen boils off when the vehicle is parked. Modern insulation materials limit boil-off losses to approximately 1 percent per parking day. Properly designed tanks are filled with liquid hydrogen leaving a gas cap where the boiled-off hydrogen can accumulate. The hydrogen demand during driving is first met from this cap. During parking, the boil-off is vented to the atmosphere only when a certain pressure in the gas cap is reached (usually above 5-6 bar). In a state-of-the-art design this would happen only when the vehicle had not been used for 4- 5 days.
Compressing hydrogen
To avoid the boil-off problem, hydrogen can also be stored at normal temperatures in cylindrical fibre or metal tanks under high pressure. Such tanks are still in the process of optimisation with respect to pressure resistance, durability, conformity and weight. Typical storage pressures are in the 200 - 350 bar range but recently storage systems up to 700 bar have become available. Since the energy consumed by compression scales logarithmically, less is used the higher the input pressure becomes. So compression from 5 bar to 350 bar consumes about 9 percent of the energy in the hydrogen while compression to 700 bar consumes only slightly more (~10 percent). If the initial pressure is 30 bar instead of 5 bar, the energy consumption will almost halve. High pressure hydrogen production technology therefore offers advantages which might outweigh its greater cost.
Other methods
Metal hydrides are another established storage method. Certain metal alloys absorb large amounts of hydrogen when exposed to the gas at certain pressures and low temperatures. Since this process is exothermic, heat is released during absorption. The hydrogen is released from the hydride by heating it. Though metal hydrides can store almost as much hydrogen as the equivalent volume of liquid hydrogen, they are so heavy that the weight of the gas is only 1 - 1.5 percent of the weight of the storage device in working systems.
Recently there has been a lot of research into new storage materials with superior qualities. For instance, highly porous activated carbon can adsorb hydrogen at cryogenic temperatures in quantities far above metal hydrides. In the best cases, it comes close to the densities of compressed hydrogen storage. Although none of the novel materials can yet replace the conventional technologies it is very likely that in the mid-term future some will achieve comparable qualities to today's methods. However, for first (and presumably even second) generation vehicles, either compressed or liquid hydrogen storage will have to be used.
Safety aspects
Special safety precautions must be taken with hydrogen. It mixes three times faster with air than natural gas and can be ignited when the air contains more than 4-5 percent of it. Moreover, ten times less energy is needed to ignite hydrogen than natural gas. However, it is more likely than natural gas to burn rather than explode as its detonation limit - the air-gas mixture which tends to explode rather than to burn - is at about 11 - 18 percent, whereas for natural gas - air mixtures it is 5 - 6 percent, only slightly above the natural gas ignition limit. This means that any ignitable agglomeration of hydrogen is likely to burn whereas natural gas is more likely to explode.
The famous accident to the "Hindenburg" zeppelin in 1935 at Lakehurst, New Jersey, was caused by the spark ignition of the aluminium paint covering the outer fabric of the zeppelin's hull - a danger known to the experts at that time. Once ignited, the hydrogen burned but did not explode. Moreover, due to three further characteristics of hydrogen, two thirds of the passengers on board survived that catastrophe.
Hydrogen is very light. This caused it to rise rather than spread out and fill the passenger cabin below the hull. Secondly, because hydrogen is the simplest molecule that exists, composed of two identical atoms, it cannot radiate heat. For physical reasons, this is only possible for more complex molecules. Thirdly, hydrogen combustion does not produce smoke in the way that burning oil does. Consequently, although the fire was not far above the heads of the zeppelin's passengers, they were not hurt by the heat or smoke. All the passengers who ran away in the short time between the zeppelin touching the ground and the break-up of the burning hull above the passenger cabin survived.
If cars or aircraft were fuelled with hydrogen today rather than gasoline or kerosene, accidents involving burning fuel would cause fewer severe injuries. Overall, experts agree that hydrogen poses no more risks than natural gas and that it might be safer than liquid fuels. Even so, safety measures including sensors and ceiling ventilation are needed to avoid an ignitable hydrogen-air mixture developing in closed rooms.
2.3 MOBILE APPLICATIONS
Because of its low weight, hydrogen was used for rockets from the beginning of space research. It even has some advantages for fuelling ordinary aircraft, permitting them to carry about 20 - 25 percent more payload. This could - at least partly - outweigh its higher cost. It also is, as we have noted, safer than kerosene, and, if produced from renewables, would cut the aircraft's carbon dioxide emissions.
However, it has to be stressed that its use would increase water vapour emissions which are mainly responsible for contrail formation and infrared reflection. The height of flights is crucial to this phenomenon. As long as an aircraft flies in the troposphere and avoids the tropopause some 8 - 12 km up depending on geographic and climatic conditions, its environmental impact would be much less harmful than with today's kerosene-fuelled aircraft.
Another broad application, if not hydrogen's most important one, will be as fuel for cars and trucks. Although their emissions will be pure water vapour, they will raise local humidity only by several parts per thousand above the normal level. But, provided the hydrogen is produced from renewable energy, no carbon dioxide, hydrocarbon and particulate emissions will be produced. Even nitrogen oxide emissions can be reduced to close to zero if better internal combustion engines are developed while electric drive systems powered by fuel cells will completely cut out all harmful emissions.
For railways, the extra space required for hydrogen fuel is not a critical parameter and electric drive systems based on fuel cells would remove the need for an overhead power supply. Even ships can be fuelled with hydrogen and some small ones have already been converted in ecologically sensitive areas. The space taken for fuel storage is not a problem here either. In short, any oil-fuelled vehicle can be converted to use hydrogen.
Although in principle hydrogen can satisfactorily replace fossil fuels in almost every stationary application, very often other non-fossil alternatives are superior. For instance, heat is better generated from solar energy or biomass burning rather than hydrogen combustion. And electricity generation by wind or photovoltaics is much more efficient than producing hydrogen, perhaps by electrolysis, and then burning it in an internal combustion engine with an attached generator, or in a fuel cell, to produce electricity again.
For a long time many people thought that the
practical use of hydrogen would only become
important when a huge storage system for electricity
was needed, perhaps around the year
2050. Apart from this, only niche applications
for hydrogen were seen, mainly in stand-alone
power systems. For instance, a house or a small
village without a grid connection might need to
store solar energy from periods with high solar
irradiation for use at other times. Storing the
power as electricity would need enormous
amounts of costly and resource-intensive batteries.
In this case, however, the conversion of
electricity to hydrogen and its conversion back
to electricity during peak power demand has
some rationale but a stronger justification
comes from the versatility it introduces as the
stored hydrogen can not only be used for reelectrification,
but also as fuel for cars or for
producing heat. It is this link between various
uses that, in a fully integrated systems view,
makes the hydrogen route superior to alternatives,
particularly now that the fuel cell is coming
into use.
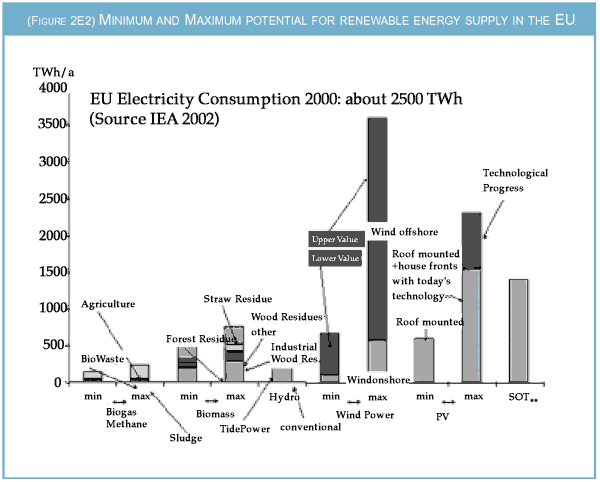 |
| This chart summarises the broad range of published and estimated possible installations of renewable
energy technologies in Europe. The biomass potential is restricted to agricultural, forestry and
municipal waste (sludge, wood etc.). The hydropower potential includes tidal power. By far the
broadest range of estimates exists for wind energy. The lower figure is based on an estimate by
Michael Grubb of the Royal Institute of International Affairs and includes offshore wind produced
at sites within 10 km off the coastline and not deeper than 10 m. The upper potential extends these
ranges to 30 km distance off the coastline and 30 m water depth. Here it should be noted that
already at least one 1GW wind park is being planned at a distance of about 60 km from the German
coastline.
The assessment of the photovoltaic (PV) potential varies widely depending on differing assumptions. The lower figure is derived assuming only roof mounted PV installations. The upper figure also includes PV installations on the facade of buildings. This estimate is based on an in-depth analysis for eight cities in the UK and an extrapolation to the whole EU by taking into account different solar irradiation as well as different building characteristics in the other regions. Two estimates are based on these figures. The first assumes that today's conversion efficiencies of about 10 per cent continue, the second that efficiency can be raised to about 13 per cent. The bar marked SOT represents the potential for building solar-thermal electric power plants in southern Europe. The chart ignores the potential for geothermal electricity production entirely. |
2.4 THE FUEL CELL - A KEY PART OF THE HYDROGEN ECONOMY
A fuel cell consists of two electrodes (highly porous carbon or another electrically conductive porous material) separated by an electrolyte. Hydrogen gas is fed to one electrode. The hydrogen molecule is decomposed into its constituents on the surface of this electrode, the electrons are stripped off and the two nuclei are separated. The electrolyte is permeable for protons (which are the positive nuclei of hydrogen atoms), but not for electrons. The protons pene trate through the electrolyte to the other electrode. There they can combine with the electrons and air (or pure oxygen) to form water vapour. Since water vapour has much less energy content than separated hydrogen and oxygen molecules, this process runs by itself. The electrons cannot penetrate through the electrolyte so they have to pass through an electric wire which is connected to the application. The flow of electrons creates the electrical current used by the consumer.
Various electrolytes have been used, some of which allow not only protons but also more complex molecules to penetrate the membrane. The electrolyte determines the specific name of a fuel cell, but the principle always is similar. This main types are the alkaline fuel cell (AFC), the proton exchange membrane fuel cell (PEM), the phosphoric acid fuel cell (PAFC), the molten carbonate fuel cell (MCFC) and the solid oxide fuel cell (SOFC).
Each of these fuel cell types has a different operating regime and application ranges. For instance, SOFC have the highest operating temperatures of about 10000C. MCFC have an operating temperature of about 6000C. and can consume carbon-rich gases like biogas directly without complicated gas purification processes being carried out first. PEM fuel cells operate at the lowest temperatures but require gas purification first. Moreover, at these low temperatures, the mobility of ions and the pace of the chemical reaction are very slow. The stripping of the electrons at the inner electrode surface needs to be initiated by expensive catalytic materials, predominantly platinum-based metals. A single fuel cell is composed of the two electrodes at each side of the electrolyte. Many individual cells can be stacked beside each other to form a fuel cell stack. The number of cells within a stack determines the power output.
Once the optimal configuration of a single cell is found, it is very simple to make many more. Fuel cell production is therefore ideally suited to mass production. This should reduce costs greatly. The first fuel cells used large amounts of platinum, making the price too high for commercial use, but successive improvements have helped to reduce the material requirements so much that it seems likely that mass produced optimised fuel cells could become relatively cheap and simple to produce.
Greater Efficiency
Fuel cell drive systems for vehicles should be much more efficient than internal combustion engines. An internal combustion engine is restricted by the laws of thermodynamics to a conversion efficiency determined by the temperatures involved in the combustion process. Consequently, on average, electricity production from heat power stations has only about 33 percent efficiency. Moreover, for cars, the efficiency is also determined by the driving situation, and this is governed by the velocity. At very low speeds - technically speaking, at partial load and low angular momentum - much energy is needed for acceleration. Since during typical driving many stops and acceleration processes are involved, the average efficiency of today's cars is very low, somewhere close to 20 percent.
Fuel cells work completely differently as
sketched in Figure 2E4.
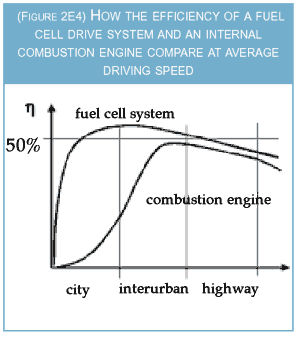 Firstly, they use an
electrochemical process, which obeys different
thermodynamical limitations so that, theoretically,
a conversion efficiency above 90 percent
can be reached. Secondly, although their efficiency
depends on the load, it is higher rather
than lower the less power is required with
respect to the maximum available power. In
other words, fuel cells are better than the internal
combustion engine for accelerating from
low speeds. Moreover, the electric drive system
has highest efficiencies at low velocities. This
helps to double the overall efficiency of a typical
driving cycle. Cycle efficiencies close to 40
percent are already being measured in today's
hydrogen cars. With technological progress,
fuel cell drive systems ought to become smaller
and cheaper. This would allow them to be oversized
which, in turn, would increase their energetic
efficiency even further.
Firstly, they use an
electrochemical process, which obeys different
thermodynamical limitations so that, theoretically,
a conversion efficiency above 90 percent
can be reached. Secondly, although their efficiency
depends on the load, it is higher rather
than lower the less power is required with
respect to the maximum available power. In
other words, fuel cells are better than the internal
combustion engine for accelerating from
low speeds. Moreover, the electric drive system
has highest efficiencies at low velocities. This
helps to double the overall efficiency of a typical
driving cycle. Cycle efficiencies close to 40
percent are already being measured in today's
hydrogen cars. With technological progress,
fuel cell drive systems ought to become smaller
and cheaper. This would allow them to be oversized
which, in turn, would increase their energetic
efficiency even further.
There is therefore considerable hope that fuel cells will completely replace today's combustion engines in almost all applications in the not too distant future. Bearing in mind the technological revolutions in other fields - such as those from transistors to microelectronics and from mainframes to small personal computers and from wired telephones to wireless small cellular phones - it can be anticipated that a fuel cell revolution could oust the old technology in one or two decades once commercialisation takes off although we are in the very early days at present.
While the fuel cell is the key technological component for using hydrogen in the transport sector and perhaps for some stationary applications as well, there are other conversion technologies which fit well into hydrogen-use strategies such as gas turbines either for fastresponse electricity generation and, maybe much more importantly, for powering aircraft.
3. HYDROGEN AND THE TRANSPORT SECTOR
General To introduce renewable energy into the transport sector various alternatives are possible. These are- direct use of electricity in electric vehicles,
- direct use of biogas (methane),
- direct use of biofuels (plant oil),
- use of ethanol from lignocellulose,
- conversion of biomass into synthetic fuels (e.g. via the Fischer-Tropsch process),
- gasification of biomass and conversion to hydrogen.
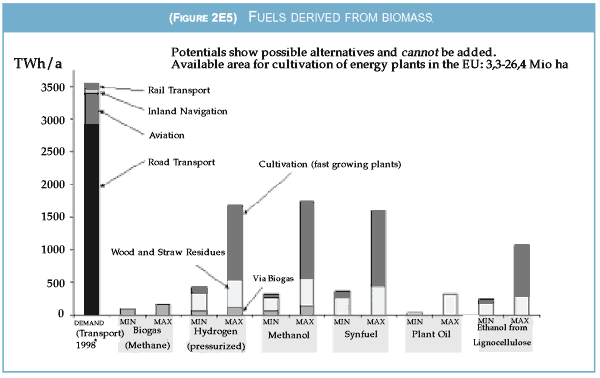
Figure 2E5 shows the potential for fuel production
from biomass sources in the EU.